ISO 11040: The Global Standards for Prefilled Syringes
Home » Services » Physical and Packaging Testing » Physical & Mechanical Testing for Medical Devices » ISO 11040 Prefilled Syringe Testing Standards
Prefilled syringes have become increasingly important in the healthcare industry, offering convenience, reduced contamination risks, and precision in drug delivery. The rise in their use, especially for biologics and vaccines, demands stringent quality and safety standards. This is where the ISO 11040 series, the international standards for prefilled syringes, play a critical role.
What is the ISO 11040 Series?
The ISO 11040 series is a comprehensive group of standards developed by the International Organization for Standardization (ISO) specifically for prefilled syringes. It outlines the design, manufacturing, and testing requirements to ensure that prefilled syringes meet the highest safety, performance, and quality standards. The series is crucial for pharmaceutical companies, device manufacturers, and healthcare providers across all of healthcare to ensure patient safety, regulatory compliance, and the reliability of drug delivery.

Need help?
Let our team of experts help you understand what’s next!
Navigating the ISO 11040 Series for Syringe Safety
The ISO 11040 series consists of eight parts, each covering different aspects of prefilled syringe design, components and testing.
ISO 11040-1: Prefilled syringes – Part 1: Glass Cylinders for Dental Local Anaesthetic Cartridges
- This part of the series defines the materials, design, dimensions and test methods for single-use glass cylinders for dental local anaesthetic cartridges and applies to primary packaging in direct contact with a drug.
ISO 11040-2: Prefilled syringes – Part 2: Plunger Stoppers for Dental Local Anaesthetic Cartridges
- This part outlines the specifications for plunger stoppers used in single-use dental local anaesthetic cartridges, including materials, dimensions, performance criteria, and labelling requirements.
ISO 11040-3: Prefilled syringes – Part 3: Seals for Dental Local Anaesthetic Cartridges
- This part addresses the material, dimensional, performance, and labelling requirements for single-use seals used in dental local anaesthetic cartridges.
ISO 11040-4: Prefilled syringes – Part 4: Glass Barrels for Injectables and Sterilised Subassembled Syringes Ready for Filling
- This part specifically addresses the requirements around materials, dimensions and test methods for glass barrels for injection preparations and sterilised subassemblies ready for filling.
ISO 11040-5: Prefilled syringes – Part 5: Plunger Stoppers for Injectables
- This part of the series outlines the specifications, including material properties, dimensions, and performance requirements, for plunger stoppers used in glass barrels for injection preparations in accordance with ISO 11040-4.
ISO 11040-6: Prefilled syringes – Part 6: Plastic Barrels for Injectables and Sterilised Subassembled Syringes Ready for Filling
- This part provides guidance on the materials, dimensions, and performance requirements for single-use polymer barrels and sterilized subassembled syringes as well as specifying components needed for sterilised subassembled syringes ready for filling.
ISO 11040-7: Prefilled syringes – Part 7: Packaging Systems for Sterilised Subassembled Syringes Ready for Filling
- This part specifies the packaging system used to deliver syringes ready for filling in tubs and nests.
ISO 11040-8: Prefilled syringes – Part 8: Requirements and Test Methods for Finished Prefilled Syringes
- This part outlines the functional and safety requirements as well as relevant test methods for single-use, terminally sterilized pre-filled syringes.

Key Aspects of ISO 11040
Patient Safety: At the heart of the ISO 11040 is ensuring that prefilled syringes deliver the required drug safely and effectively, whether that is a biologic or anaesthesia. Design and material specifications minimize the risk of contamination, leakage from the primary packaging, or mechanical failure of a vital function. This is especially crucial for vaccines, biologics, and anaesthesia where even minor deviations can impact the efficacy of the drug or lead to adverse reactions / harm to the patient.
Maintenance of Sterility:
One of the key challenges with prefilled syringes is maintaining the sterile barrier and drug stability throughout its intended shelf life. The different parts of ISO 11040 ensures that the materials used in the syringes or components are compatible with the drugs they contain and maintain the sterile barrier.Dimensional Specifications:
Across the ISO 11040 series, guidance is provided on the dimensions and tolerances for the syringe and its components. This ensures that syringes produced by different manufacturers can function properly with various drug products, components or accessories. A precise dimensional design is also important to ensure correct dosing, maintenance of the sterile barrier and connectivity with administration devices.Mechanical Strength and Integrity:
Prefilled syringes must withstand the rigors of transportation, storage, and use. The requirements for the mechanical strength of the syringe barrel, the needle, and the sealing system help to ensure that the syringe will not break, leak, or fail to function during normal use or accidental drops.
The Importance of ISO 11040
Importance of ISO 11040 for prefilled syringe Manufacturers
For pharmaceutical and medical device companies, who are designing new syringes or syringe components, the ISO 11040 series is a very useful place to start when in the design phase or the verification and validation phases. Guidance is provided on ancillary testing that needs to be considered, such as detecting endotoxins and particulates, deliverable volume and biological requirements (ISO 10993-1).
Supplementary Standards and Guidance
While the ISO 11040 Series works well on its own, there are other standards and guidance that can supplement the content of the series.
USP<382> Elastomeric Component Suitability in Parental Product Packaging and Delivery Systems
- Provides functional suitability requirements of delivery systems, including primary packaging components, that are partially or completely made of elastomeric material.
ISO 80369-7 & -20: Small-Bore Connectors for Liquids and Gases in Healthcare Applications
- While not specific to syringes, ISO 80369 (parts 7 & 20) apply to medical devices that use small-bore connectors, which may include prefilled syringes for drug delivery. These standards help reduce the risk of misconnections that could lead to adverse outcomes and patient harm.
ISO 23908: Sharps Injury Protection
- This standard provides requirements test methods for evaluating the performance of sharps injury protection features.
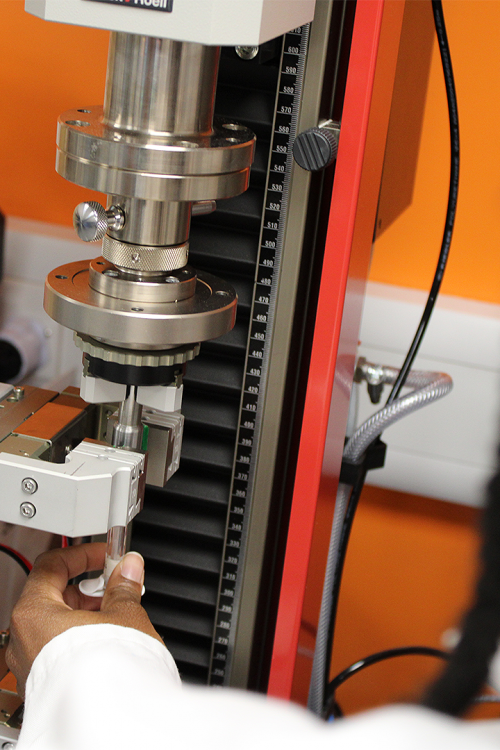
Why Think Cormica When You Think ISO 11040

Cormica’s deep expertise in medical device testing, combined with our unwavering commitment to quality and precision, ensures that clients receive the most reliable, accurate, and compliant results. With ISO 11040 standards at the forefront of the pre-filled syringe market, choosing a trusted partner is crucial. Cormica Laboratories offer end-to-end testing solutions that not only meet regulatory requirements but also enhance product safety and performance, allowing faster and safer product launches.
Our global presence, equipped with state-of-the-art laboratories, supports businesses at every stage, from development to market readiness. By partnering with Cormica, clients gain access to a team dedicated to continuous improvement and the highest ethical standards, ensuring your pre-filled syringe products are tested with the utmost integrity and precision. We are not just a testing service provider—we are a partner in your product’s success.
Let Cormica’s expertise in ISO 11040 guide you through the complexities of regulatory compliance and product quality assurance. Think Cormica, and trust in a partner that’s committed to improving patients’ lives by providing comprehensive testing services.
