Container Closure Integrity Testing (CCIT)
Home » Services » Physical and Packaging Testing » Ageing, Transit and Packaging Testing » Container Closure Integrity Testing (CCIT)
Ensure Product Safety & Compliance with Advanced CCIT Solutions
We provide comprehensive Container Closure Integrity Testing (CCIT) services to ensure the sterility, safety, and regulatory compliance of pharmaceutical and medical device packaging.
Our CCIT Services
Cormica offers a suite of CCIT methods tailored to meet industry standards:
- Hydrogen Trace Gas Detection (In-house Method) – Highly sensitive, non-destructive testing.
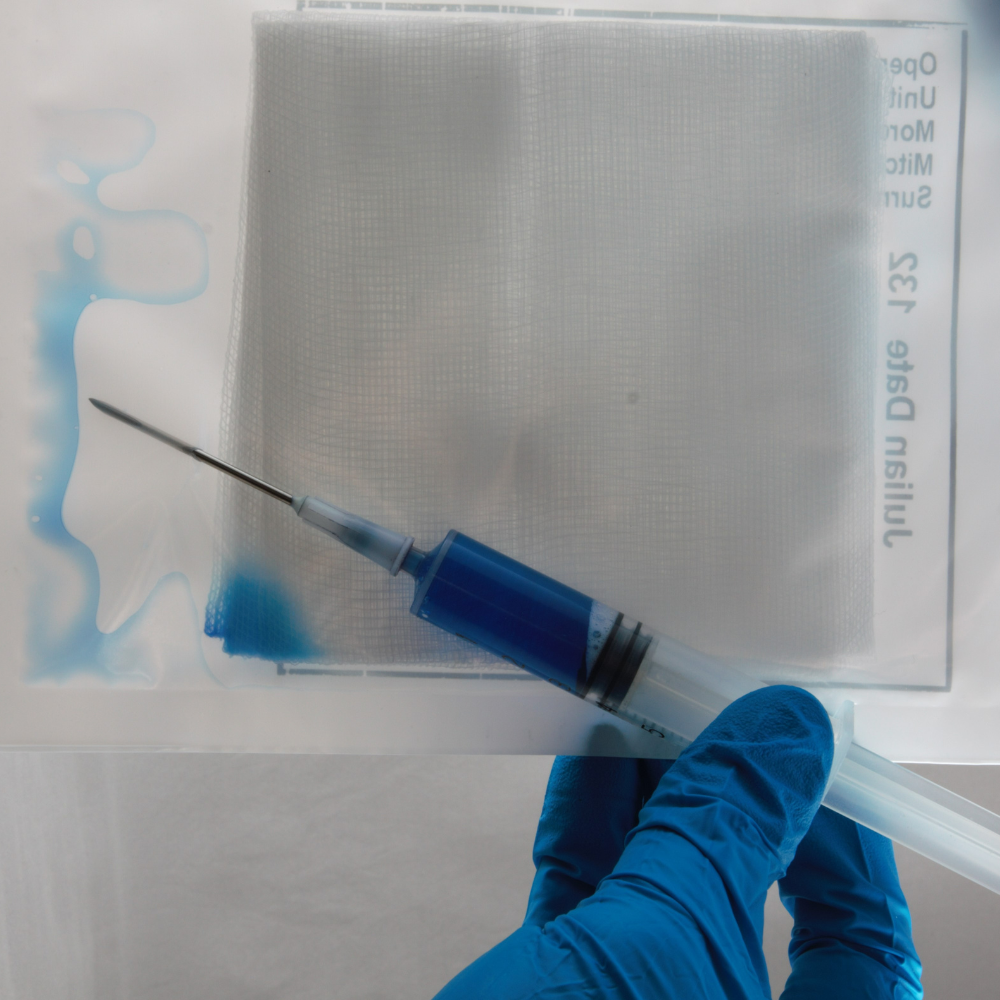
- Blue Dye Ingress Test – Detects leakage in flexible and rigid containers.
- Sterility Testing – Ensures microbial integrity of parenteral products.
Why CCIT is Essential
Regulatory agencies worldwide, including the FDA, EMA, and WHO, require pharmaceutical and medical device manufacturers to demonstrate the integrity of their packaging systems. CCIT is vital to:
- Ensure Sterility – Prevent microbial contamination.
- Maintain Product Stability – Avoid chemical degradation due to leaks.
- Meet Regulatory Compliance – Adhere to international safety and quality standards.
- Reduce Risk of Recalls – Mitigate costly product failures and liability issues.
Compliance & Regulatory Standards
Our CCIT services comply with global regulatory requirements, ensuring your products meet the highest industry benchmarks:
- USP <1207> – General guidance on CCIT for parenteral products.
- ISO 11040-4 – Pre-filled syringes (PFS) integrity testing.
- ISO 8871-5 – Elastomeric parts for parenteral packaging.
- ISO 8362-2 – Injection containers and accessories.
Why Choose Cormica for CCIT?
- Global Coverage
- Efficient & Flexible Testing
- Regulatory Expertise
- Advanced Methodologies
Get in Touch
Ensure your pharmaceutical and medical device packaging meets global safety and regulatory standards. Contact Cormica today to discuss your CCIT requirements and experience our expert testing solutions.
