Physical, Functional & Packaging Testing
Physical, Functional & Packaging Testing for Medical Devices, Combination Products and Pharmaceuticals
Accelerate your medical device’s path to market with Cormica’s rigorous Physical & Packaging Testing solutions. Our experts validate your device’s functionality, durability, packaging integrity, and safe transport, ensuring product performance, patient safety, and regulatory compliance throughout development and distribution.
Testing Excellence You Can Trust
Cormica’s Physical & Packaging Testing services are conducted in accordance with ISO 17025 standards. This accreditation underscores our dedication to reliable data, robust testing protocols, and compliance with industry-leading practices.
Request a call back from our team to learn how Cormica can safeguard your product’s success.

Our Device Testing and Packaging Validation Solutions
Physical & Mechanical Device Testing
Design Verification & Validation Testing
Needle Injection Systems
- Design Verification Testing
- ISO 11608
- ISO 11040
- ISO 23907-1
- ASTM D6653 Air Transport Simulation
- ISO 80369
Parental Containers
- ISO 11608-3
- ISO 8871-5
- ISO 11040-8
- USP 1207
- Dye Solution Tightness
- Trace Gas Detection
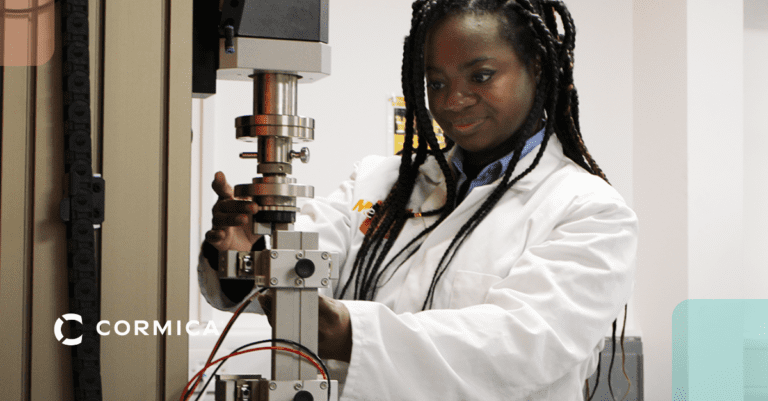
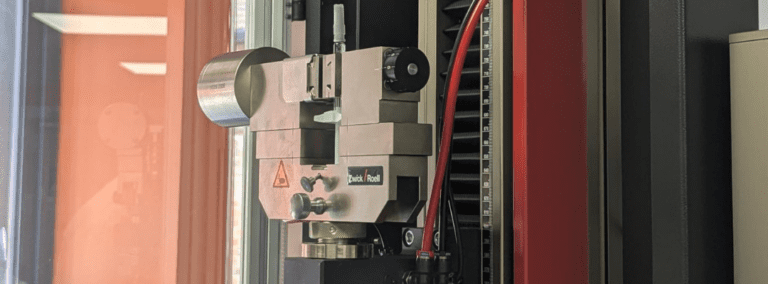
Physical Testing / Mechanical Testing
- Tensile & Compression Tests
- Pressure & Vacuum Tests
- Flow Rate
- Attachment & Detachment Forces
- Pre-Conditioning
- ISO 80369
- Safety Feature Confirmation
- Sharps Bins
- ISO 23907-1
- Torque & Axial Forces
- Penetration Tests
Device Stability Studies
- Real Time & Elevated Conditions
- ISO 11608
- Tensile & Compression Tests
- Attachment & Detachment Forces
- Torque & Axial Forces
Ageing, Transit and Packaging Testing
Accelerated Ageing & Real Time Ageing:
- ASTM F1980 – Standard Guide for Accelerated Ageing of Sterile Barrier Systems and Medical Devices
Transit Simulation Testing:
- ASTM D4169 – Performance testing of shipping containers and systems
- ASTM D7386 – Performance testing of packages for single parcel delivery systems
- ISTA 3A – Packaged products for parcel delivery system shipment (70kg/150lb or less)
Packaging Testing:
- ASTM F1886/F1886M – Visual inspection for flexible packaging
- ASTM F88/F88M – Seal strength of flexible barrier materials
- ASTM F1140/F2054 – Burst testing of flexible package seals
- ASTM F1929 – Detecting seal leaks in porous medical packaging by dye penetration
Meeting Global Standards for Medical Device & Packaging Testing
Cormica’s Physical & Packaging Testing services adhere to the most stringent regulatory requirements and industry best practices, including:
Device Performance & Safety Testing Standards:
- ISO 11608 Series (Needle-based injection systems)
- ISO 11040 Series (Prefilled syringes)
- ISO 80369 Series (Small-bore connectors for liquids and gases)
- ISO 7864 (Sterile hypodermic needles)
- ISO 11607-1 and ISO 11607-2 (These critical standards focus on packaging for terminally sterilised medical devices.)
- EU Medical Device Regulation (MDR) (Ensures compliance with the MDR’s stringent requirements for safety, performance, and documentation throughout your device lifecycle.