Biological Indicator Testing
Home » Services » Microbiology Testing » Biological Indicator Testing
We offer Biological Indicator (BI) Testing services through our trusted global laboratory network, ensuring that your sterilisation processes meet regulatory requirements and deliver consistent, reliable results.
What is Biological Indicator (BI) Testing?
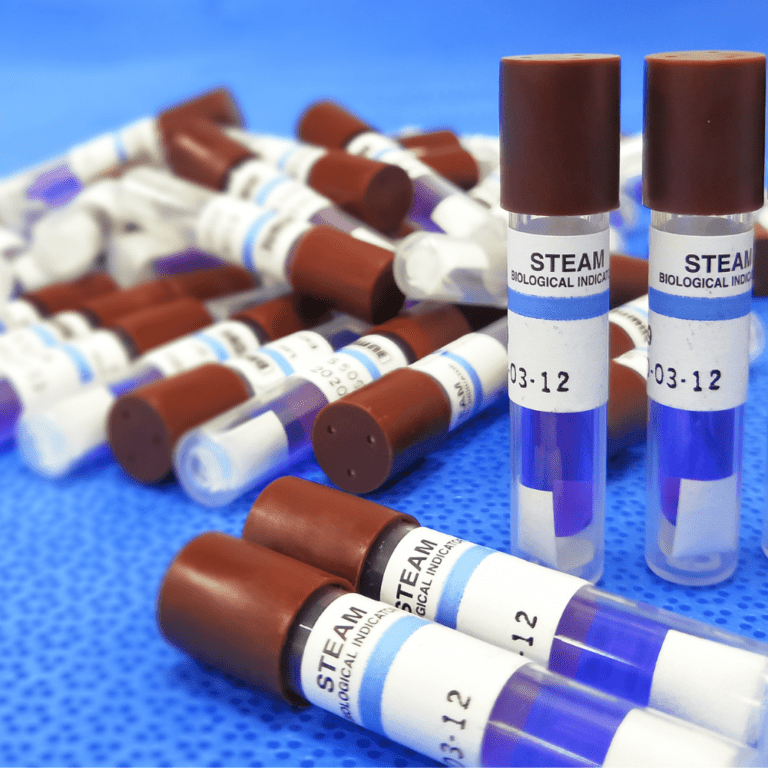
BI Testing is an essential part of validating and monitoring sterilisation processes. Biological indicators contain highly resistant microorganisms that are used to test the efficacy of sterilisation methods, including:
Steam sterilisation
Ethylene oxide (EO) sterilisation
Dry heat sterilisation
Hydrogen peroxide sterilisation
The presence or absence of microbial growth after sterilisation provides clear evidence of whether the sterilisation cycle effectively eliminates harmful microorganisms.

Our Testing Process
Sample Preparation: Biological indicators are prepared and exposed to the sterilisation process.
Incubation: Samples are incubated in optimal conditions to detect microbial growth.
Analysis and Reporting: Results are analysed, and a comprehensive report is provided, detailing compliance and any necessary corrective actions.
Partner with Cormica for Reliable Testing Services
Get Started with BI Testing Today
Ensure the reliability of your sterilisation processes with Cormica’s BI Testing services. Contact us today to learn more or to request a quote. Our team is ready to assist you in achieving the highest standards of safety and compliance.

