X-Ray Powder Diffraction Services
Home » Services » Analytical Chemistry » X-Ray Powder Diffraction Services
We are a trusted partner for X-Ray Powder Diffraction (XRPD) analysis, delivering rapid, accurate and high-quality data to support pharmaceutical and medical device development. Our laboratories are GMP, GLP and ISO 17025 accredited, ensuring the highest standards in scientific integrity and regulatory compliance.
With expert scientists, advanced instrumentation and flexible capacity across our UK, US and EU facilities, we provide fast turnaround times without compromising precision. Whether you need support during early-stage development, regulatory submissions or routine release testing, Cormica offers a reliable, XRPD solution tailored to your needs.
X-Ray powder diffraction (XRPD) analysis provides critical information regarding the solid form of your product, supporting drug discovery and development, regulatory submissions, formulation development, batch release and storage stability. X-Ray powder diffraction is quick, non-destructive, adaptable and very widely applied, and can test as little as 5 mg of sample.
Characterisation by X-Ray powder diffraction (XRPD) is fundamental to determining key physical properties such as polymorphic form, crystallinity and amorphicity, plus phase identification, phase purity and phase quantification.
Want to Understand More About How X-Ray Diffraction Works? Read Our Blog “X-ray Diffraction: A Simple Approach”


Specific Tests and Analysis
The Role of X-Ray Diffraction in Drug Development
NCE drug discovery and development typically takes many years to bring a molecule to the market, costing billions of dollars in the process. With each phase of development, the depth and breadth of analytical understanding and data required increases, encompassing identification of the molecule, specific characteristics, assay and purity, inorganic and organic impurities, specific physical & chemical properties, and storage stability, for the active ingredient (API), non-active ingredients (excipients), and the finished product.
Generic drug development short cuts this process, with the emphasis on demonstrating equivalence to the originator product via the Abbreviated New Drug Application (ANDA) process. Despite the shortened nature of this approach, a significant amount of evidence is required to support a claim of equivalence, covering many of the same areas as for an NCE.
X-ray diffraction analysis and the understanding / control of solid form is critical to both development tracks, playing a key role in:
- Understanding and benchmarking the physical form of the API
- Phase purity
- Solid form optimisation
- Salt and polymorph screens
- Polymorphic risk assessments
- Crystallinity / amorphicity assessment
- Formulation development
- Pattern deconvolution for complex mixtures
- QC Batch release testing
- Storage stability
Learn more about XRPD testing services
At the fundamental level understanding / benchmarking the solid form of the active ingredient (or other critical component) helps demonstrate that both the manufacturing process and the active ingredient itself is under control. This could include determining if the API is crystalline or amorphous, or confirming if the same form is generated each time the API is produced, at lab scale, at pilot scale, and at production scale.
Many solid materials, such as pharmaceutical intermediates, APIs and final products, can exhibit polymorphism – the ability of a solid to express more than one crystalline form – and different polymorphs of the same material can exhibit startlingly different properties, from melting point and hydration state, to dissolution performance, long term physical stability and bioavailability. Hence, understanding the behaviour of your product with respect to solid form is of key importance – and a regulatory expectation.
XRD can be used to understand this behaviour, as each polymorph – or pseudo-polymorph, hydrate, solvate, co-crystal, or salt form – will demonstrate a unique diffraction pattern. This facilitates the monitoring of any polymorphic or solid form changes that may occur during development, scale up, manufacturing or during storage, and also enables optimisation of solid form, by allowing for a close understanding of the form being expressed. Learn more about solid form optimisation.
Moreover, XRD is the key technique utilised to carry out polymorphic risk assessments, polymorph and salt screening studies, phase purity assessments, and batch release testing for solid form.
Methodologies can be qualitative (simple pattern matching), function as limit tests for undesired components or be fully quantitative.
Our service offering includes XRD method development, pre-validation, validation and method transfers, plus storage stability studies.
Physical stability of the solid form within a suspension product is of great interest to regulators. Suspensions are dynamic systems, and depending on the formulation, the solids may effectively dissolve and reprecipitate many times over the shelf life of the product. Hence it is of great importance to understand whether the solid form is maintained consistently during this process.
Benchmarking the diffraction patterns of the API, excipients, and placebo formulation, and comparing to the patterns generated by both fresh and aged suspension formulation samples facilitates the monitoring of solid form, and a risk assessment to be completed. The methodology and understanding developed as part of the risk assessment process can also be applied to finished product storage stability / shelf-life studies.
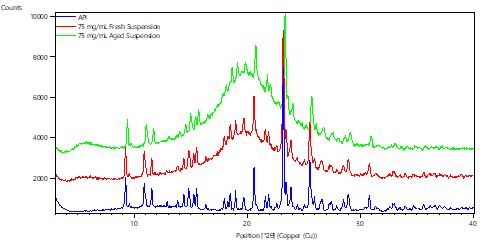
Comparison of Diffraction patterns for Fresh and Aged Oral Suspensions with that of Reference API
Understanding, benchmarking and controlling the degree of crystallinity / amorphicity of the active ingredient is essential in terms of demonstrating that the manufacturing process – and the product itself – is under control. Degree of crystallinity / amorphicity has an impact on multiple API characteristics, but more importantly, variability or uncontrolled changes in the degree of crystallinity can impact dissolution and dissolution rate, and hence drug bioavailability.
Additionally, while the amorphous phase can present many advantages in terms of drug development – enhancing the performance of low solubility compounds, for instance – such phases are often relatively unstable in the longer term; as high energy solids and have a tendency to recrystallise. Hence, they must be very closely controlled and monitored, and their stability demonstrated, to be acceptable to regulators.
XRPD analysis enables the qualitative and quantitative assessment of the degree of crystallinity of a powder sample. By utilising the relationship between crystallinity and pattern intensity, and controlling for factors such as particle size and morphology, indicative comparisons may be made between samples of the same type.
Quantitative methodologies require the preparation of calibration standards; our micronising, milling and blending capability enable the preparation of reference materials and calibration blends. Learn more about powder processing.
These are highly energetic processes which can impact both surface and bulk properties, for instance by generating microscopic regions of amorphicity, which then have a tendency to recrystallise over time.
XRD, along with a range of particle and powder characterisation techniques can be used to assess the impact of such powder processing, including assessing crystalline / amorphous content and polymorphic form before and after processing.
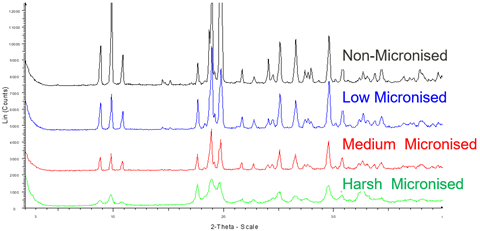
Reduction of Sample Crystallinity with Increasing Micronisation Time / Energy
Exposure to moisture can have a significant effect on some powdered APIs and finished products, necessitating, for instance, specific handling requirements or protective packaging configurations.
XRD, when used before and after controlled exposure to moisture during Dynamic Vapour Sorption (DVS), can be used to understand the impact on solid form and crystallinity levels.
Relaxation studies – the exposure of an amorphous or partially amorphous sample to moisture at a defined % relative humidity and temperature, for a defined period of time – are of particular importance when understanding the stability of the amorphicity. Here, XRD is used as a fast and reliable way of understanding amorphicity levels, and the aging / relaxation of the sample.
Characterisation and physical properties determination form an important part of QC release testing, and for active ingredients, excipient and finished products where solid form has been demonstrated to be an important parameter to be controlled during the development cycle, the release process ideally includes XRD analysis.
XRD methods for use in batch release must have undergone stage appropriate verification or validation; this would typically include system suitability, specificity, repeatability precision, and intermediate precision, with linearity, accuracy, LOD / LOQ included as appropriate for quantitative methods. According to ICH Q2 R2 robustness testing would be completed and documented as part of the method development process.
Storage stability of both active ingredients and finished products typically requires assessment of chemical and physical stability, with XRD analysis for physical form stability sitting alongside an appropriate moisture content technique such as Karl Fischer titration or loss on drying.
XRD plays an important role in the testing of many different medical device-based products, for properties such as crystallinity, phase purity and stoichiometry.
Cormica has extensive experience of establishing and validating such methods, including routine release testing on a fast turnaround basis, working to standards such as ISO 13779, Implants for surgery — Hydroxyapatite.
The HighScore Plus software allows for complex pattern deconvolution via the Rietveld refinement approach. This enables phase quantification of complex samples via a whole pattern matching approach; additionally, Rietveld refinement can be used for analysis of crystallite size based on the change in profile parameters, compared with those of a standard sample.
HighScore Plus also includes access to industry standard crystal structure databases, enabling comparison of your sample patterns with premium quality reference patterns.
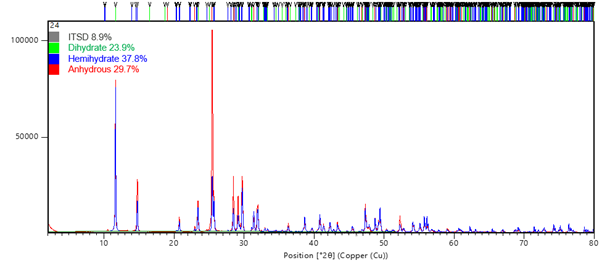
Phase Quantification of Mixed Hydrated Forms via Rietveld Refinement
Almost every powder diffractometer is configured to use reflection geometry as standard. This is where the incident beam and the detector are on the same side of the sample surface. This geometry works very well for inorganic materials that can be prepared as flat samples with randomly oriented crystallites. However, it can be challenging to accurately measure reflections at low angles using this configuration, and great care must be taken regarding the sample preparation, to ensure a smooth, flat surface exactly flush with the sample holder surround. Moreover, preferred orientation can result in sub-optimal data quality.
Cormica can also offer transmission geometry, which is a straightforward approach whereby the incident beam and detector are located perpendicular to one another, with the sample in between. This configuration facilitates low angle measurements, minimises preferred orientation effects, and enables the analysis of sample types such as wafers and thin films, which are challenging to measure in reflection mode. This configuration is highly applicable to the low-absorbing organic compounds such as those used in the pharmaceutical industry.
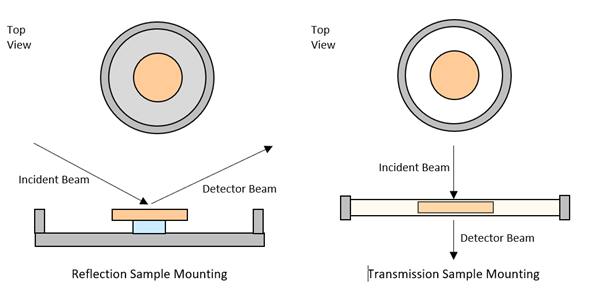
Reflection versus Transmission Sample Mounting
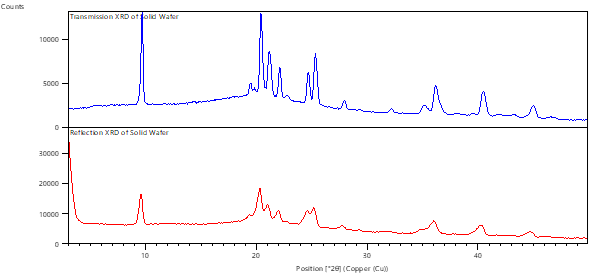
Improved Resolution and Peak Sharpness for Low Density Wafers using Transmission Geometry
Instrumentation
- Malvern Panalytical Empyrean Series 3 X-Ray diffractometer with PIXcel3D detector, reflection and transmission geometries, in situ non-ambient analysis and high-throughput measurements for multi well-plates. 42-position sample changer.
- Bruker D8 Advance X-Ray diffractometer with LynxEye™ detector, reflection geometry, and 9-position sample magazine.


