Formulation Development
The team at Cormica can meet your formulation needs, developing “phase – appropriate” formulations tailored to the characteristics of the API, for NCEs and generics alike.
offer a fast, cost effective and flexible approach to formulation development of drug product:
- The team have considerable expertise of developing and testing formulations from simple to complex (e.g. powders, solids, liquids, creams, gels, slow release, fast release) across a range of delivery routes (oral, nasal, inhalable, injectable, topical).
- The team works alongside material science, DMPK investigators and toxicologists to design an effective formulation screening programmes to de-risk your programme and solve challenges with formulation development.

Drug Product Development
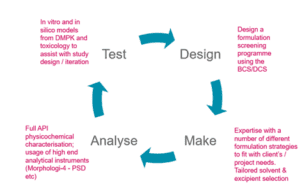
More Analytical Chemistry:
Analytical Chemistry Resources
Drug Product Development
Analytical techniques typically deployed for Q1/Q2/Q3 equivalence studies include:
- XRPD
- FTIR
- DSC, mDSC, TGA
- Isothermal Microcalorimetry (Amorphicity and relaxation studies)
- BET Specific Surface Area
- Helium Pycnometry
- Spraytec
- Morphologi 4-ID / Morphologically Directed Raman Spectroscopy
- Particle size / charge
- NGI (Next Generation Impactor)
- Inverse Gas Chromatography
- Size Exclusion Chromatography
- HPLC / uHPLC
- UV, PDA, DAD, Fluorescence
- ELS, RI, CAD, MS, MS/MS
- Inductively Plasma – Optical Emission Spectroscopy / Mass Spectrometry
IVBE (in vitro Bioequivalence) studies to FDA recommendations for a range of dosage forms including:
- Nasal sprays and nebulizers
- Dry powder & metered dose inhalers
- Topical creams & ointments
- Ophthalmics
- Liposomes
- Oral solid dose
Identification / structural elucidation of impurities / synthesis of unknown impurities in the API and generic prototypes finished products:
- Preparative Mass-Directed Impurity Isolation
- LC-MS/MS
- NMR
Method development and GxP capabilities:
- Analytical method development, verification, validation and transfer
- GMP release testing of Generic API and critical excipients
- We are GMP and GLP accredited; FDA inspected