CLEANING VALIDATION AND VERIFICATION
Cormica provides an unrivalled breadth of analytical solutions, techniques and applications to the pharmaceuticals, medical device, healthcare, agrochemicals, biocides, food and feed sectors, including pesticides. We offer a comprehensive service supporting cleaning validation and ongoing cleaning verification for our customers.
Cleaning Validation and verification
The cleanliness of manufacturing and packaging equipment, and the production facility itself, in the pharmaceutical and agrochemical industries (and others), is critical in preventing product to product cross-contamination, ensuring operator, patient and end-user safety. Manufacturers must ensure the absence of unwanted residues of active, inactive, or detergent ingredients before the facility is used to manufacture a second product.
During manufacturing process validation (or retrospectively), companies must demonstrate that the cleaning procedure routinely employed in the facility limits potential carryover to an agreed acceptable level. Subsequently, when in full production, the company must verify that the validated cleaning procedure has been carried out effectively and the equipment and facility are sufficiently clean to permit the introduction of a different product to the facility.
Ensuring Safety Through Cleaning Validation: A Comprehensive Approach
Cleaning validation is the methodology used to assure that a cleaning process removes the residues of the previous product/cleaning agents to predetermined levels. This includes risk assessing the product constituents to determine those which present a carryover risk, as well as the development and validation of suitable analytical methods. This is a one-off exercise.
Cleaning verification is the process used to ensure that the validated cleaning process has been executed effectively. This can be by visual inspection and/or analytical verification, after taking surface swabs or rinses, and is carried out each time the facility switches products.
Our Medical Device & Combination Product Testing Services
- Analytical Chemistry
- ANDA Services for Generic Pharmaceutical Development
- Contaminants & Foreign Bodies
- Extractables and leachables
- Formulation Development
- GXP Analytical Method Development, Validation and Transfer
- Nanoparticle Sizing
- Occupational Hygiene Monitoring
- Out of Specification Investigations Analytical Chemistry
- Particle / Powder Properties & Characterisation
- Pharmacopeial & Monograph Methods
- Physicochemical Testing
- Product Safety Registration
- Rheological Characterisation
- Solid Form Optimization
- Solid State Analysis
- X-Ray Powder Diffraction Services
Our Analytical Chemistry Resources
Comprehensive Approach to Testing and Validating Cleaning Processes
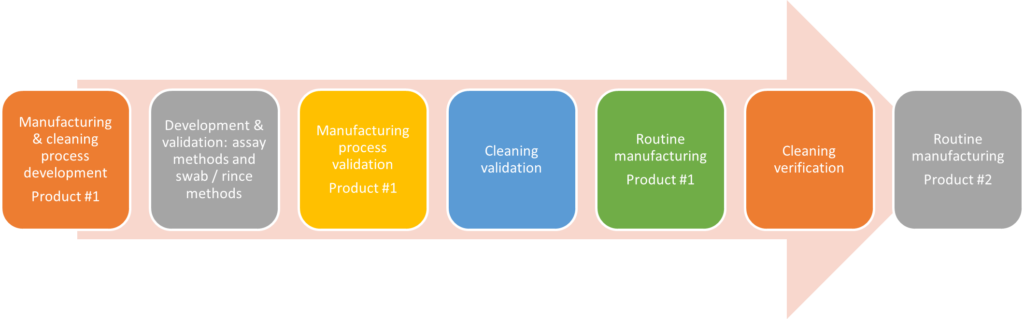
Cormica’s wide range of analytical equipment, including uHPLC, uHPLC-MS, ICP and TOC, as well as our extensive experience in this area, can be applied to both the development and validation of cleaning methods, and to the ongoing testing of cleaning samples, taken by swabbing or rinsing of equipment, for instance.
Specific analytical procedures and methods, cleaning limit calculations and protocols must be developed and agreed with the client for each cleaning study/target contaminant and will be dependent on a wide range of factors including the surfaces to be cleaned, the nature of the analyte and the limits required.
- Calculation of cleaning limits, using the Maximum Allowable Carryover (MACO) calculations
- Assessment of the surface materials which require cleaning
- Development and validation of an appropriate analytical assay method:
- For active and inactive ingredients, HPLC-UV or uHPLC-UV. uHPLC-MS is also available
- For detergents and cleaning agents, HPLC-ELSD or uHPLC-CAD
- For a non-specific method, covering all carbon-containing entities from active ingredients, through detergents, to organic rinse solvents, Total Organic Carbon is often the best choice.
- An elemental marker can be assayed using ICP-OES or ICP-MS.
- The method must be sufficiently sensitive for the calculated limits.
- Development and validation of the swabbing/rinsing procedure:
- Selection of the swabs
- Selection of the swab extraction matrix/volume
- Swab recovery from relevant coupons (e.g. stainless steel, glass, specified plastics)
- Section of rinsing medium, appropriate rinse volumes
- Analyte recovery from rinses
- Development of the equipment cleaning protocols
- Sampling and analysis of swab samples
- Calculation of results using appropriate safety factors for swab recovery and surface area swabbed
- Reporting results in an agreed format
Cormica's Cleaning validation and verification Capabilities
- GMP and GLP accredited; FDA inspected
- Analytical method development, verification, validation and transfer
- Swab method validation
- Development of cleaning protocols, cleaning limits
- Site visits for cleaning risk assessment and subsequent sampling
- Training of customer staff in swabbing techniques
- Analytical Methods for Cleaning Validation / Verification
- HPLC / uHPLC
- UV, PDA, DAD, Fluorescence
- ELS, RI, CAD, MS, MS/MS
- Total Organic Carbon
- Inductively Plasma – Optical Emission Spectroscopy / Mass Spectrometry
- HPLC / uHPLC