Cormica Enhances ISO 11040 Syringe Testing with MET Acquisition of Z2.5kN Zwickline Machine
Type:
Published:
Author:

Steven Malbon
Combination & Physical Devices Manager
Medical Engineering Technologies
Request More Information
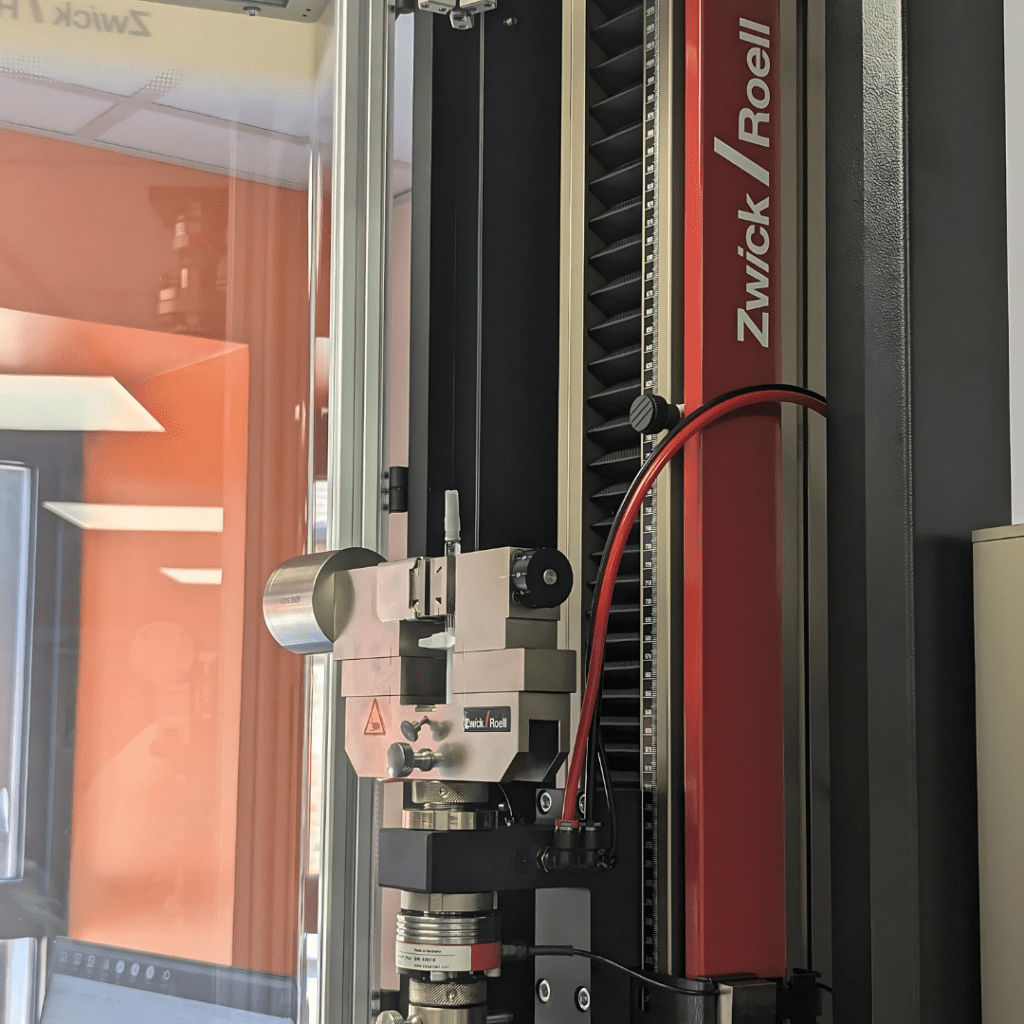
Cormica has enhanced its ISO 11040 pre-filled syringe testing with the acquisition of another new Z2.5kN Zwickline testing machine.
Located at our Dover testing site, Medical Engineering Technologies (MET), the Zwickline features dual testing areas, a comprehensive set of accessories specifically designed for ISO 11040 testing and a safety shield to ensure Scientists can work safely.
The Z2.5kN Zwickline will increase our throughput and capacity. Its dual test areas allow us to utilise a single machine for testing that previously required two separate test stands. This innovation not only streamlines our operations but also enhances efficiency, enabling us to conduct a higher volume of tests in a shorter amount of time.
With our constant commitment to improvement and data integrity, the testXpert III software supplied with the Z2.5kN Zwickline is fully compliant with CFR21 Part 11. This compliance ensures that our testing processes adhere to stringent regulatory requirements for data integrity.
With ISO 11040 testing being an integral part of our services, the inclusion of accessories specifically designed for this purpose enhances ease of use and broadens the range of syringes that can be tested. This specialised equipment ensures precise and reliable testing, tailored to meet the specific requirements of ISO 11040 standards. Additionally, the Z2.5kN Zwickline’s modular design allows for the integration of various accessories, expanding our testing capabilities even further. This flexibility ensures we can meet diverse client needs and stay at the forefront of testing technology.

How Does This Help Our Clients?
- Increase to throughput and capacity: Allows us to be more efficient, cost-effective and scalable while enhancing client satisfaction and our ability to meet the varying needs of our clients
- CFR21 Part 11 compliance: This is crucial for meeting regulatory requirements, ensuring data integrity and maintaining quality assurance.
- Modular design for expansion of scope: This provides us with great versatility, space efficiency, future-proofing and enhanced capabilities.
- Enhanced safety features: Health & Safety is at the forefront of everything we do and the addition of the in-built safety shield shows our commitment to the safety of our Scientists.
Other Cormica News, Events & Resources
Contact Us
Ready to take the next step? We’d love to hear from you. Contact us today to discuss your testing needs and find out how Cormica can help you achieve your goals.








