ISO 11040-4:2024 - Latest Updates and Requirements
Type:
Published:
Author:

Steven Malbon
Combination & Physical Devices Manager
Medical Engineering Technologies
Request More Information
With the release of the fourth edition of ISO 11040-4:2024, it officially rescinds and replaces the third edition, ISO 11040-4:2015. This updated version introduces several significant changes and enhancements to the standard, incorporating the latest industry practices and providing improved clarity throughout. The main changes to the standard include:
Update to general requirements for quality systems, testing and documentation:
Documentation and activities within the standard must now adhere to a formal Quality Management System (QMS). Test equipment must be qualified, and test methods validated. All documentation should be retained according to the QMS, and both electronic records and handwritten signatures related to validation, process control, or other quality decision-making processes shall be documented.
Specific requirements for staked needle syringes added:
Needle diameter and length as well as the design of the finger flange will be agreed between the manufacturer and the client. A table has been added for tolerances on the various combinations of syringes volume and needle length. Additionally, figures illustrating typical staked needle syringe designs have been included.
Specifications for finger flange breakage resistance, front end breakage resistance and penetration force added: Flange breakage resistance and front end breakage resistance (previously Luer cone breakage resistance) now have specified minimum force to break values. A specification has now been provided for the maximum force for a needle top penetrate a test foil, this is to help detect needle point and lubrication defects that have an impact on patient comfort.

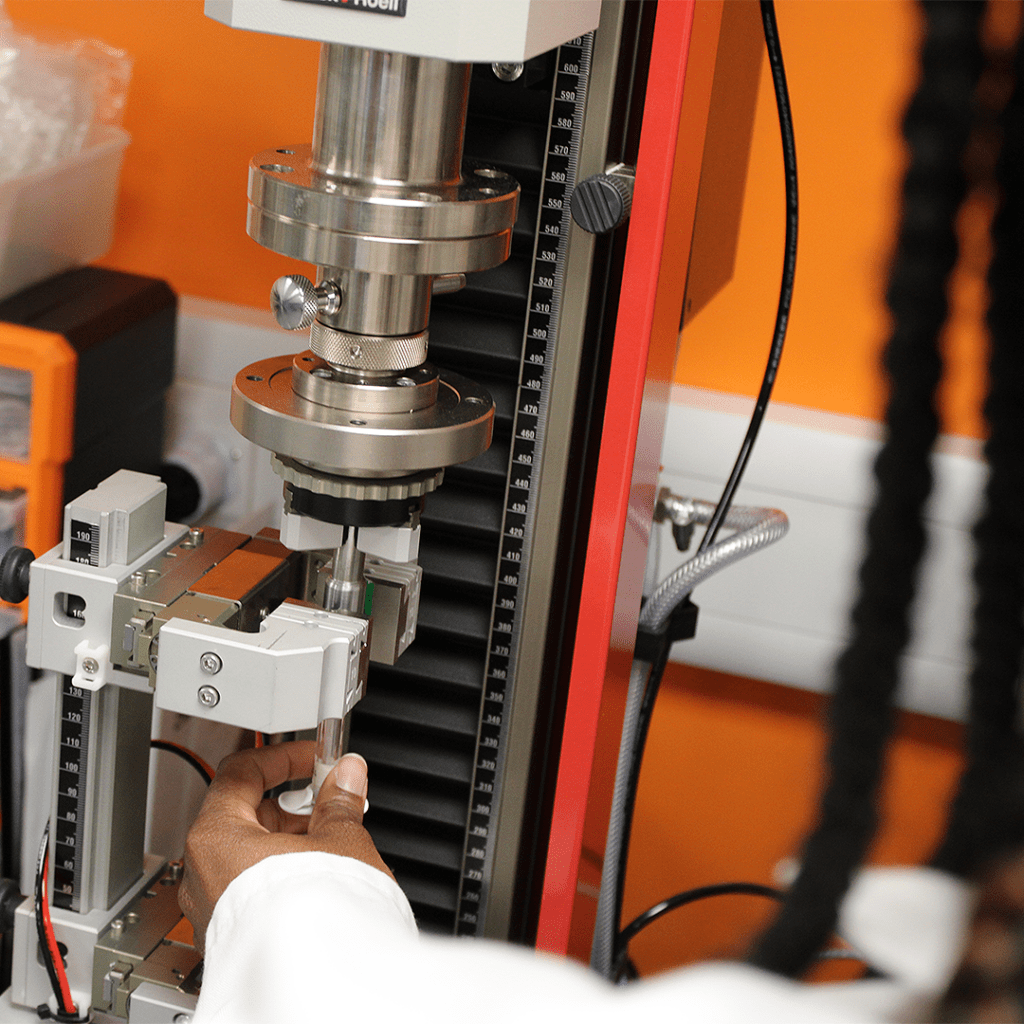
Requirements for the performance of non-lubricated syringes added: Under the section for Glide Force Test Method, there is now details on using the glide force test for non-lubricated syringes. Previously the method was only applicable to lubricated syringes.
Figures throughout the standard updated: Figures through this new version of the standard have been updated for visual and technical clarity.
Annex B: The content of Annex B has been updated to show additional components for a sub assembled syringe ready for filling, this includes plunger stoper variants and plunger rod examples. Previously, Annex B included models of different head designs for the glass barrel of syringes.
Other Cormica News, Events & Resources
Contact Us
Ready to take the next step? We’d love to hear from you. Contact us today to discuss your testing needs and find out how Cormica can help you achieve your goals.








