Bacterial Endotoxin Testing White Paper: Understanding Methods to Ensure Product Safety
Endotoxins, are part of the outer membrane of gram-negative bacteria. Lipopolysaccharide and lipoproteins make up the Endotoxin complex, and they are released when intact gram-negative bacteria is distrupted and their outer membrane disintegrates, triggering the toxicity mechanism of the fractionated LPS. Endotoxin testing must be carried out to ensure patient safety. Not only end-product medical devices or injectables should be tested, but also on the raw materials that are used to make them.
Despite this requirement, endotoxin testing is often overlooked, and many manufacturers may rely too much on sterility testing. As described in the harmonized European, United States, and Japanese pharmacopeias (2-4), sterility testing will detect live microorganisms, including Gram-negative bacteria. If these bacteria are destroyed during the sterilization process, however, endotoxin will be released and will remain undetected.
Type:
Published:
Immune response to exposure
Failure to detect endotoxins can have harmful, or even fatal, effects. When bacteria and endotoxin enter the bloodstream, they trigger an immune response, usually inflammation, the release of cytokines such as interleukin 1, and the production of tumor necrosis factor. The release of cytokine signals cause neutrophils to migrate toward the point of infection. This migration usually leads to phagocytosis of all associated organisms and proteins. When the host’s immune system is weak, or a high level of infection is encountered, the bacteria can cause sepsis and its associated risks.
The dangers of endotoxin exposure
The importance of endotoxin testing is clear when looking at how susceptible and sensitive humans are to even minute amounts of endotoxin. Endotoxins may be introduced into the bloodstream through contaminated intravenous devices or medications. Typically, products must demonstrate less than 5 Endotoxin Units (EU) per Kg of patient body weight. For intrathecal injection, the specified endotoxin limit is less that 0.2 EU/Kg of patient body weight Exposure to the compounds results in fever, inflammation, and endotoxemia. When left untreated, it has the potential to cause sepsis (5), resulting in septic shock, which is responsible for 44,000 deaths in the United Kingdom (6) and approximately 200,000 deaths in the US (7) each year.
Ensuring that equipment and medication are free from endotoxin is particularly important when caring for vulnerable patients, including the elderly, those in intensive care, and infants. For example, sudden infant death syndrome (SIDS) has been linked to varying levels of endotoxin present in the bloodstream (8).
It has also been shown that endotoxin exposure can damage nerve axons directly or indirectly (9), suggesting that it is an essential factor in the pathogenesis of critical illness polyneuropathy (CIP) in sepsis. This article describes and compares the analytical methods used to detect bacterial endotoxins.
Currently, detection methods include the rabbit pyrogen test, described in European Pharmacopoeia (Ph. Eur.) 9.0 2.6.8. and United States Pharmacopeia (USP) <151> (2,3), which consists of injecting live rabbits with the injectable product and monitoring for an increase in body temperature.
Alternatively, in-vitro tests such as the monocyte activation test (MAT) and methods that use limulus amoebocyte lysate (LAL) are widely relied on for the detection of bacterial endotoxin. LAL is an aqueous extract of amoebocyte cells found in Limulus polyphemus, the Atlantic horseshoe crab. The main methods that use LAL are gel-clot, Kinetic quantitative chromogenic LAL, and Kinetic quantitative turbidimetric LAL.

Gel-Clot Method
The gel-clot method is the simplest and oldest LAL test and is used to detect the presence or absence of endotoxin in the prepared sample. When endotoxin encounters the LAL, it initiates cascade of enzymatic reactions. The cascades results in the production of at least three serine protease zymogens: Factor C, Factor B and a proclotting enzyme (10). These enzymes alter the amoebocyte coagulogen present in LAL to form a detectable gel-clot.
LAL testing should always be performed to the test manufacturer’s recommendations. Usually, testing is performed by adding equal parts reconstituted LAL to prepared test sample in a 10- x 75-mm soda lime glass reaction tube. Test material should be processed (e.g., using a vortex mixer), for at least 30 seconds, and then placed in an equilibrated heating block or water bath at a temperature usually 37 ± 1 ºC.
After inserting the first tube, the timer should be set for 60 minutes (or suitable time the LAL manufacturer has validated and recommended) and the tube left undisturbed until the incubation time has elapsed. While samples are tested, a standard curve with at least four known endotoxin values must be drawn to prove the sensitivity of the LAL (Table I).
| Table I: Gel-clot standard curve graph. The symbol λ is the sensitivity of the lysate used. A standard curve is considered valid when the end-point falls between 2λ and 0.5λ. | |||
|---|---|---|---|
| 2λ | λ | 0.5λ | 0.25λ |
| + | + | - | - |
| + | + | - | - |
If a clot has formed and remains intact at the bottom on the tube when fully inverted, this is a positive, showing that the concentration of endotoxin is equal or greater than the sensitivity of the LAL used. When no clot forms or the clot breaks when inverted, this is a negative result, showing that the amount of endotoxin in solution is less than the sensitivity of the LAL used. This method if a semi-quantitative method at detecting endotoxin as multiple dilutions can be tested and the endpoint used to calculate the approximate endotoxin level.
The gel-clot method is thought to be the most sensitive and accurate LAL test, giving fewer false positives and being less susceptible to interference than other methods. USP <85> states that “in the event of doubt or dispute, the final decision is made based upon the gel-clot limit test unless otherwise indicated in the monograph for the product being tested”
Despite this, the method it a time-consuming process due to there being no automated procedure, which also requires an operator to read and interpret the test results, adding potential for bias or human error. Several factors can affect the results obtained, including but not limited to:
- pH disruption (i.e., when samples in test solution are not within the stated 6.0-8.0 pH range) (2)
- Presence of chemical inhibitors such as ethylenediaminetetraacetic acid (EDTA)
- Viscosity of the product, because naturally viscous products could create a clot like structure in the tube
- Denaturing agents such as a strong acid or base, a concentrated inorganic salt, or an organic solvent
- Base alcohol molecules, which can react with the LAL and precipitate out of solution.
Chromogenic Method
Kinetic and End-Point Chromogenic
The chromogenic assay was developed after it was determined that the haemocyte lysate od a horeshoe crab would hydrolyze specific amino acid sequence containing the chromophore pNA (P-nitroanilide) (11) This method uses synthetic chromogenic substrate containing a specific amino acid sequence, which mimics the cleavage site in coagulogen. Endotoxin-activated LAL cleaves this site, causing the release of pNA, which gives the assay its distinctive yellow colour.
The pNA molecule absorbs light at the specified and validated wavelength, and the chromogenic assay measures the absorbance of light at the wavelength of 405nm. The degree at which light it absorbed is directly proportional to the amount of endotoxin within the sample.
Testing is performed in duplicate, by adding equal parts of LAL to sample and then incubating at 37 ± 1 ºC. Both sample and reagent are placed in an absorbance microplate reader, and the reaction is automatically monitored over time for the appearance of a predetermined absorbance change. The absorbance of light at 405 nm is continuously monitored throughout the incubation period. The reaction time change is inversely proportional to the amount of endotoxin present; a log/log correlation between the reaction time and the endotoxin concentration is plotted. The concentration of unknown samples can then be calculated from the standard curve which is run alongside the sample.
For the kinetic method, the concentration of endotoxin in a sample is calculated based on its reaction time compared with the reaction time of the endotoxin standard curve (Figure 1).
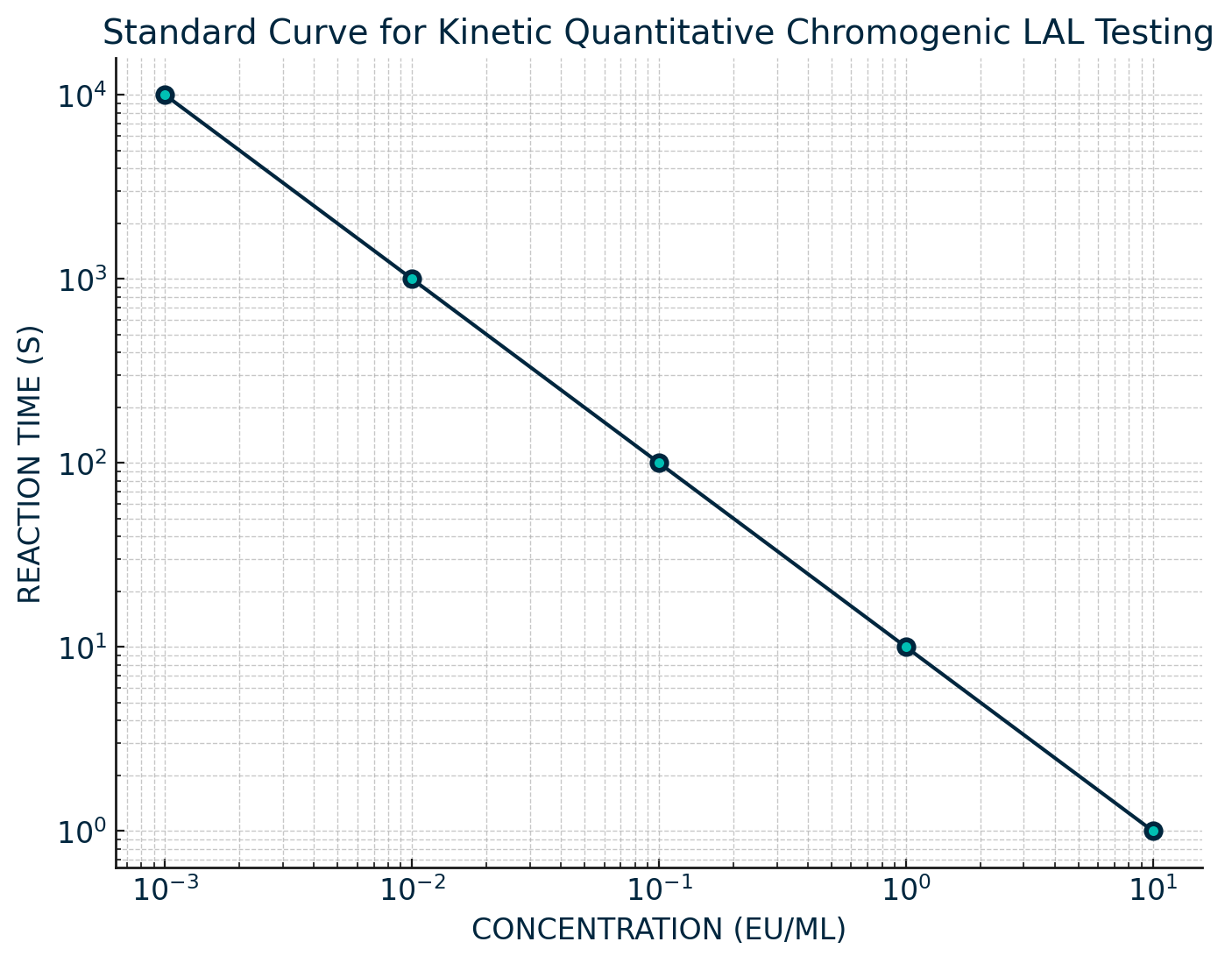
The end-point-chromogenic method reads the value of endotoxin in each sample only after a predetermined incubation period has elapsed. Both methods use a standard curve that requires a minimum of three known endotoxin concentrations.
The chromogenic method can be automated, which decreases the amount of time to required to perform a test, allowing for more samples to be analysed per unit of time. The chromogenic method is user friendly, and calculation can be performed simply.
Results by this method can vary due to the wide range of the coefficient of variant (CV), which measures the precision in testing. Results for this method are only valid when CV values are ≤10% or ≤25%, depending on the requirements set by the LAL vendor. The higher the CV% value is, the more variation there is between the two test replicates, meaning a lower level of precision for the result.
As the chromogenic method is so user friendly it is often the ‘go to’ method for many labs. Due to the sensitivity of the test means that interference can be an issue, for example products that denture proteins, bind endotoxin, and cause precipitations. When exposed to LAL, sometimes samples can turn a specific colour, which can result in interference in the test results. For example, some serine protease may yield false positives, simply due to their colour in solution.
Turbidimetric method
The Turbidimetric method is a photometric method which measures the rate of turbidity development caused by the reaction between the LAL and Endotoxin (2). It is an automated, modified extension of the gel-clot test. The modified LAL reagent is altered to contain a lower quantity of coagulogen, resulting in a turbid mixture unable to form a solid clot when exposed to endotoxin (12). The advantage of using the turbidimetric method over the simpler gel-clot method is that it gives a quantitative result, showing the level of endotoxin in the sample solution, and the sensitivity of the LAL used is lower.
In this approach, an equal mixture of sample and reconstituted LAL should be incubated at 37 ± 1 ºC, and the turbidity or optical density (OD) of the mixture can be measured at each specified and validated wavelength continuously throughout the incubation period. The reaction time (i.e. ,the time required for the mixture to reach onset OD) is inversely proportional to the amount of endotoxin present.
The end-point-turbidimetric method is performed in the same way as the end-point-chromogenic method. The result given by the turbidimetric method can be altered when testing blood, plasma, serum, and similar materials, but it is particularly sensitive to interference from suspended or turbid products, which can result in false positives.
As discussed, no method is perfect, and each has its advantages and disadvantages. Many scientists believe the gel-clot method yields the most accurate result when determining if endotoxin is present in a sample (12). Because the method is known to interact with fewer materials, its results are less likely to have been affected by inhibition or enhancement from the sample. Despite this fact, the amount of time that is required to prepare samples using this process has made it less popular for use in raw material testing in some laboratories
Results by this method can vary due to the wide range of the coefficient of variant (CV), which measures the precision in testing. Results for this method are only valid when CV values are ≤10% or ≤25%, depending on the requirements set by the LAL vendor. The higher the CV% value is, the more variation there is between the two test replicates, meaning a lower level of precision for the result.
As the chromogenic method is so user friendly it is often the ‘go to’ method for many labs. Due to the sensitivity of the test means that interference can be an issue, for example products that denture proteins, bind endotoxin, and cause precipitations. When exposed to LAL, sometimes samples can turn a specific colour, which can result in interference in the test results. For example, some serine protease may yield false positives, simply due to their colour in solution.
Conclusion
As discussed, no method is perfect, and each has its advantages and disadvantages. Many scientists believe the gel-clot method yields the most accurate result when determining if endotoxin is present in a sample (12). Because the method is known to interact with fewer materials, its results are less likely to have been affected by inhibition or enhancement from the sample. Despite this fact, the amount of time that is required to prepare samples using this process has made it less popular for use in raw material testing in some laboratories
The most significant advantage of the kinetic methods over the gel-clot method is their ability to extrapolate a quantitative result. Being able to extrapolate results can be invaluable when testing raw materials because it can offer insights into potential sources of endotoxin contamination. The Kinetic method is a high yield, user-friendly method but interacts with many different compounds. As a result, validation using these methods can be time consuming.
An additional consideration is that one of the biggest factors that can cause analogous results is technician error. Until the endotoxin testing process can be fully automated, with minimal human interference, subjectivity may affect the interpretation of results.
The need for proper training
The best, and only, way to approach endotoxin testing is to try different methods, ensure that technicians are well trained in each one, and are fully aware of the strengths and limitations of each procedure, as well as the potential difficulties that individual samples may pose, and that due care and attention is taken at every stage.
Despite issues with some test methods, endotoxin testing is crucial to ensuring product quality and patient safety. Its importance as a quality control tool should never be overlooked.
All methods described in this article have been approved by Ph. Eur. 9.0 2.6.14, USP 40 <85>, and JP XVII 4.01. for medical devices, injectable materials, and the raw materials used to make them.

References
- X. Wang and P.J. Quinn, “Endotoxins: Lipopolysaccharides of Gram-Negative Bacteria,” In: X. Wang and P.J.Quinn P. (eds) Endotoxins: Structure, Function and Recognition. Subcellular Biochemistry, Vol 53. (Springer, Dordrecht), 2010.
- European Directorial for the Quality of Medicines and Healthcare, European Pharmacopoeia 9th ed, (Strasbourg, Council of Europe, 2016).
- US Pharmacopeial Convention, United States Pharmacopeia 40. (Rockville, MD, US Pharmacopeial Convention, 2017).
- Pharmaceuticals and Medical Devices Agency, Japanese Pharmacopoeia XVII, (Tokyo, Pharmaceuticals and Medical Devices Agency, 2016).
- S.M. Opal et al., (Eds): Endotoxemia and Endotoxin Shock: Disease, Diagnosis and Therapy. Contrib Nephrol. Basel, Karger, vol 167, pp 14-24, 2010. (DOI:10.1159/000315915)
- University of York, “Sepsis Could Cost UK Economy up to £15.6 Billion Each Year, New Study Suggests,” www.york.ac.uk, Accessed Sept. 22, 2017.
- H. Wang, et al., “National Variation in US Sepsis Mortality: a Demographic Study,” International Journal of Health Geography, biomedcentral.com, February 15, 2010.
- N. Sayers et al., Journal of Clinical Pathology, 49(5), pp.365-368, 1996.
- B. Mohammadi et al., Journal of Neurology, 255(2), pp.265-272, 1996.
- T. Sandle, Pyrogens, Endotoxin and the LAL Test: An Introduction in Relation to Pharmaceutical Processing, Global BioPharmaceutical Resources Newsletter, pp1-16, 2012, [Accessed 28th Sep. 2017].
- T. Morita et al., Jpn J Med Sci Biol. 31(2):178-81, 1978. PMID: 682375
- T.Joiner, P.Kraus, and T.Kupiec, International Journal of Pharmacutical Compounding, Vol. 6(No. 6), pp.408-409, 2002.
