Pre-Filled Syringe Design Verification Testing Webinar
Discover the critical processes behind design verification testing for pre-filled syringes in this expert-led webinar, Pre-Filled Syringe Design Verification Testing, presented by Zhane’ Healey and Hollie Gladwin. Join us to gain a comprehensive understanding of the testing processes that ensure the safety, functionality, and regulatory compliance of pre-filled syringes.
What You’ll Learn:
This session will provide valuable knowledge on key aspects of pre-filled syringe design verification, ensuring your devices meet regulatory and performance standards.
- Introduction to Design Control
Learn how to navigate the design control process for pre-filled syringes, including defining user needs, intended use, and indications for use. Understand how these inputs shape the development process. - Establishing Design Inputs and Outputs
Gain practical insights into specifying and documenting design inputs (user requirements) and creating tangible design outputs like engineering schematics, device blueprints, and component specifications. - Verification Testing
Understand the methods for verifying whether design outputs meet the specified design inputs. We’ll address key questions such as, “Was the device built right?” and provide real-world case studies to illustrate successful verification. - Regulatory Insights and Case Studies
Examine key regulatory guidelines and lessons learned from recent industry examples, including device recalls and compatibility issues, to highlight the importance of rigorous verification & validation testing. - Interactive Q&A Session
Bring your questions to our experts and receive tailored advice specific to your challenges and projects.
Why Attend?
By attending this webinar, you’ll be equipped with the tools and knowledge to confidently manage design verification testing for pre-filled syringes, ensuring your devices meet the highest standards for performance and compliance.
You’ll gain insights into the critical role that rigorous testing plays in ensuring device safety, reliability, and patient satisfaction. The session provides a detailed exploration of global regulatory standards and compliance requirements, offering practical strategies to align your processes with these benchmarks.
Cormica’s mission is to improve patients’ lives by providing comprehensive testing services, enabling clients to launch and release their products safely and rapidly across the world.
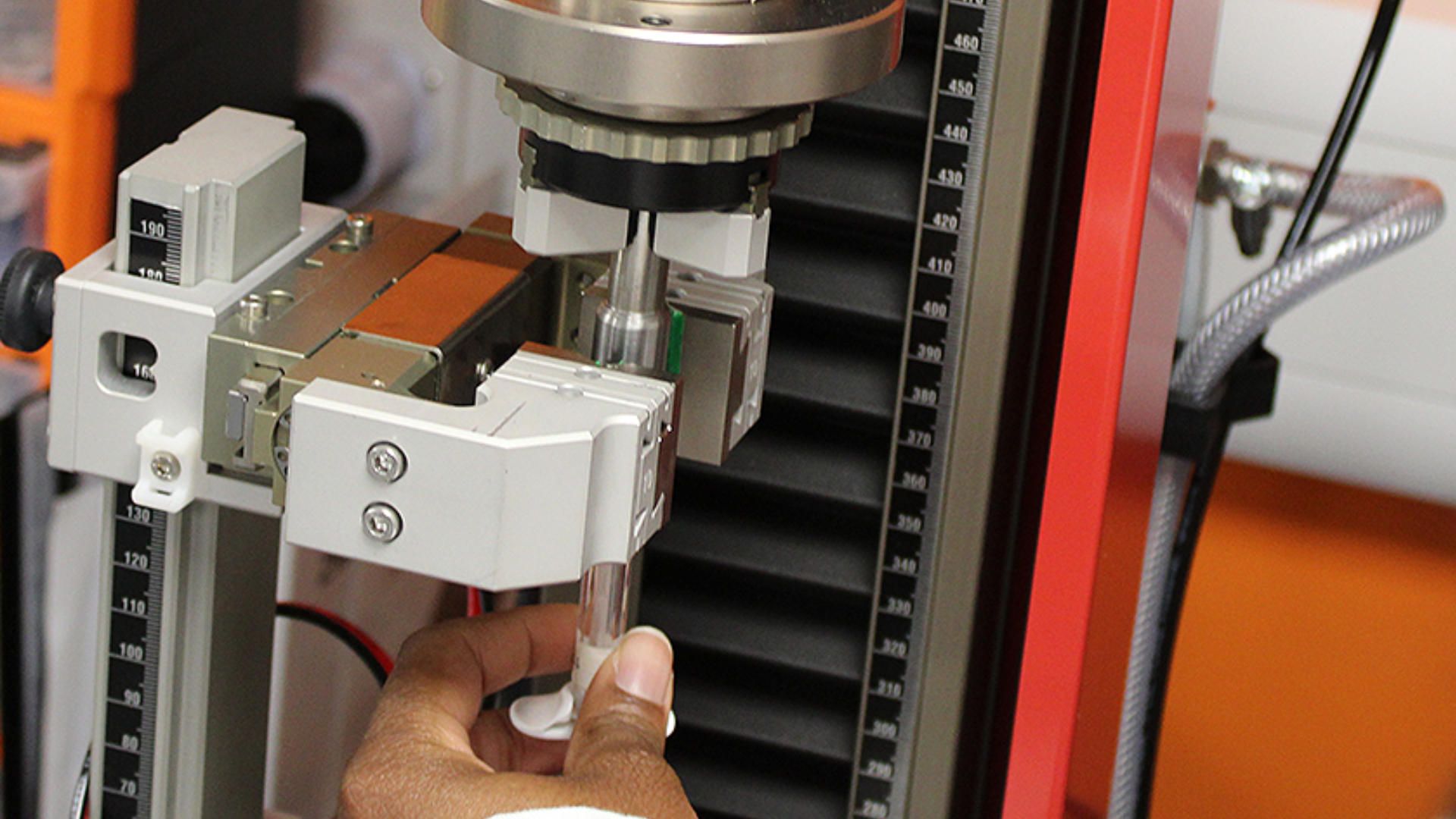
Pre-filled syringe testing is essential for ensuring the quality, safety, and functionality of drug delivery systems, aligning with ISO 11040 standards and regulatory expectations.
Cormica provides a comprehensive range of pre-filled syringe testing services, covering everything from break loose and glide force to plunger movement and component integrity assessments. These tests are designed to validate that your syringe systems maintain consistent performance and support safe, effective drug delivery.
With Cormica’s experienced team and advanced testing capabilities, you can be confident in meeting industry standards. Whether you’re developing new syringes or optimising existing products, contact us today to discuss your testing needs at sales@cormica.com
Download Webinar:
Type:
Authors:
Zhané Healey is an experienced Team Leader with over five years in the laboratory industry. She currently leads the Combination & Physical Devices testing team at Medical Engineering Technologies Ltd, a role she has excelled in for the past three years. Zhané holds a Double Grade Distinction from the University of Greenwich’s Advanced Apprenticeship program in Biology Technician/Biotechnology Laboratory Technician.
Hollie has a strong foundation in science and healthcare, with 13 years of experience in commercial pharmacy and a Certificate of Higher Education in Biomedical Science from the University of Portsmouth. Currently working as a Scientist, she specialises in CCI testing and the use of Cormica MET’s Zwick Roell Testing apparatus. Her career has been defined by adaptability, attention to detail, and a passion for continuous improvement.


