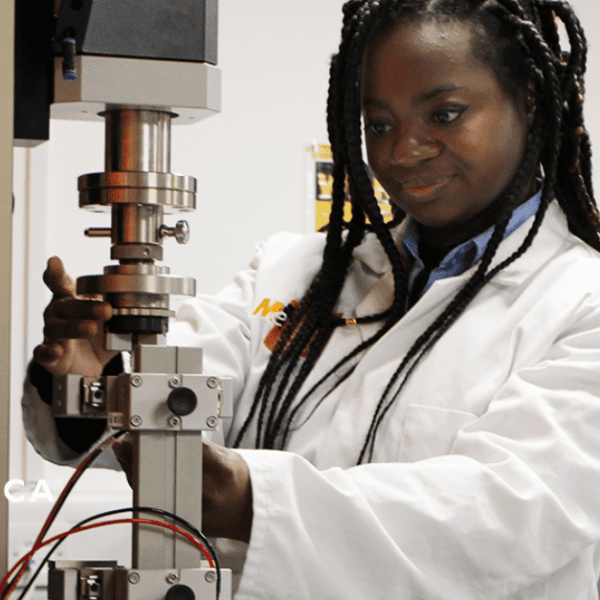
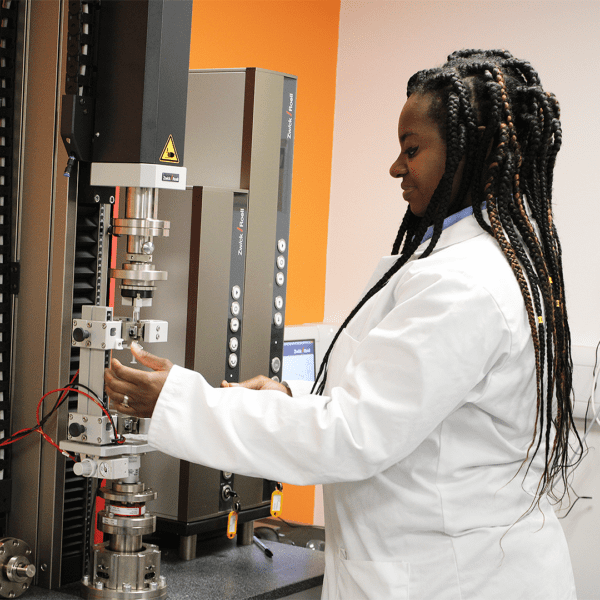
Medical Device Design Verification and Validation
Why Design Verification and Validation (DVT) Matters
In the highly regulated world of medical devices, ensuring compliance with global standards like FDA, MDR, and ISO is critical. Design Verification Testing (DVT) and Design Validation Testing are key processes that confirm your device meets both user needs and regulatory requirements, ensuring patient safety and device efficacy.
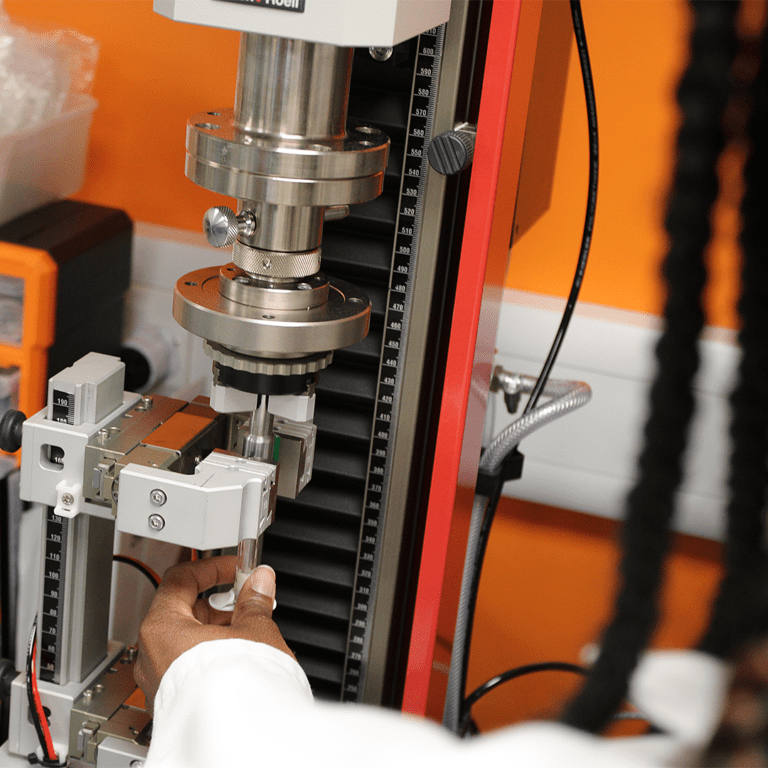
Ensuring Compliance, Safety, and Efficiency Through Rigorous Testing

Services We Offer:
Design Verification Testing (DVT):
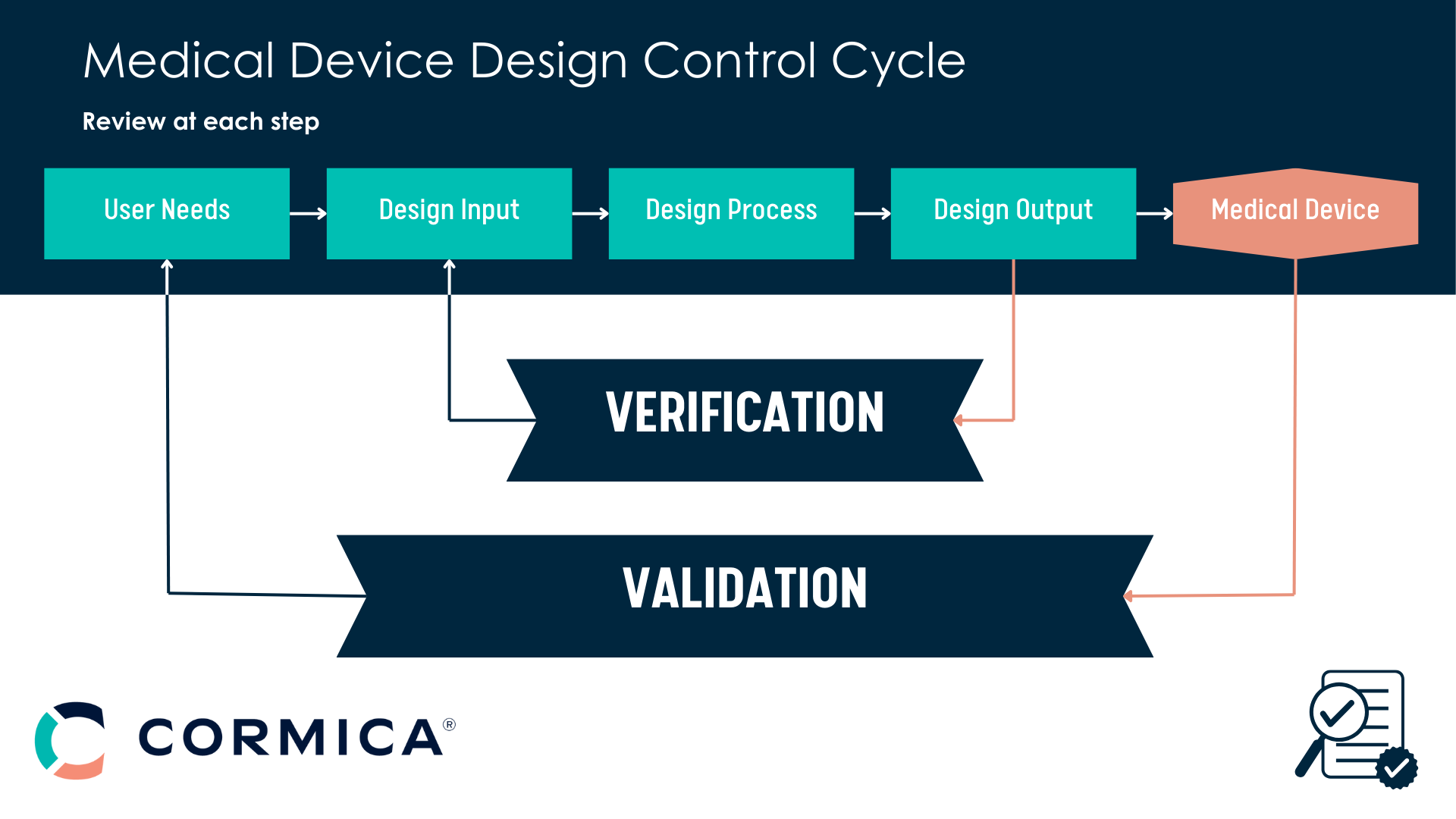
Verifying that your device design outputs meet the specified design inputs. This ensures “the device was built right.”
Methods include:
- Functional Testing
- Physical and Chemical Testing
- Compatibility Analysis
Design Validation Testing:
Validating that your device meets its intended use through evidence-based assessments. This confirms “the right device was built.”
Examples include:
- Usability Studies
- Clinical Simulations
- Final Product Testing
Regulations We Address:
- FDA Design Control Guidelines
- ISO 13485 Compliance
- MDR and IVDR Requirements
- GMP and GLP Standards
Why Choose Cormica?
- Expertise Across Medical Device Categories:
From pre-filled syringes to wearables and orthopedics, our technical scientists bring deep subject-matter expertise. - Regulatory Focus:
We prioritise compliance to avoid costly recalls and patient safety risks, as seen in cases like the Ethypharm Aurum syringe recall. - Cutting-Edge Testing Facilities:
Accredited labs equipped with the latest technology to meet and exceed industry standards.
Medical Device Design Verification vs Design Validation
Medical Device Design Verification & Design Validation Frequently Asked Questions
Design Verification Testing (DVT) is the process of ensuring that a medical device’s design outputs meet the specified design inputs. This involves rigorous testing methods, such as functional, physical, and compatibility analysis, to confirm that “the device was built right.” DVT is essential for compliance with global regulations like FDA and ISO 13485.
Design Validation Testing ensures that a medical device meets its intended use by validating it in real-world scenarios, often involving usability studies and clinical simulations. In contrast, Design Verification Testing focuses on whether the design outputs align with the design inputs. Together, these processes ensure the device is both safe and effective.
Design Verification and Validation are mandatory for meeting global medical device regulations, including FDA guidelines, ISO 13485, and MDR requirements. These processes prevent product recalls, improve patient safety, and ensure devices perform as intended, which are critical for gaining regulatory approval.
Design Verification and Validation are essential for a wide range of devices, including pre-filled syringes, auto-injectors, wearable devices, orthopedics, cardiovascular implants, and more. Any device regulated under FDA, MDR, or ISO standards must undergo these processes to ensure compliance and efficacy.