Physical, Functional & Mechanical Medical Device testing
Ensuring Performance, Safety, and Compliance
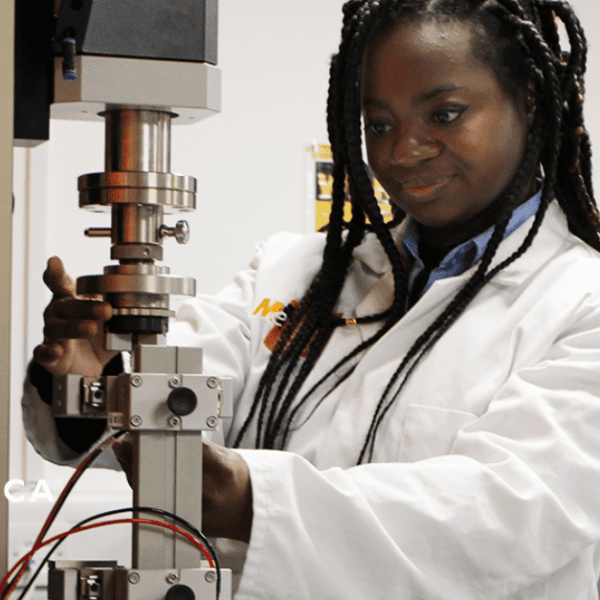
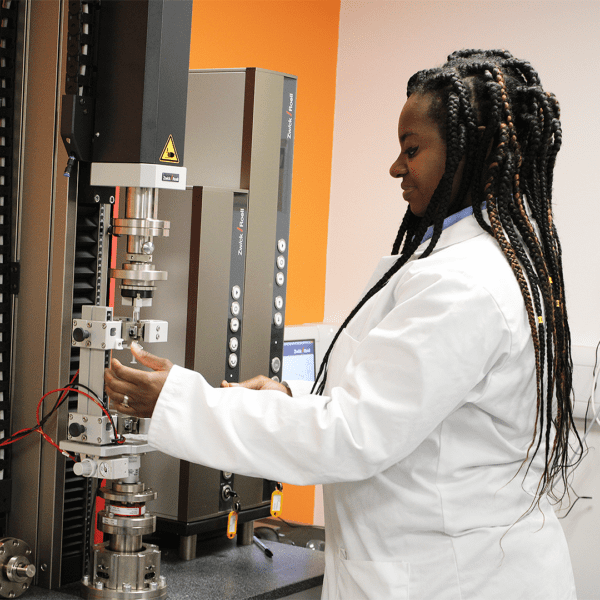
Reliable performance and safety are paramount when it comes to medical devices and Combination Devices. Our Physical & Mechanical Testing services are designed to rigorously evaluate every aspect of your product, from durability and strength to the stability and functionality of critical components.
By adhering to industry-leading standards such as ISO 11608, ISO 11040, and ISO 80369, we provide comprehensive testing solutions that ensure your devices meet the highest regulatory and quality expectations.
Whether you need design verification or validation testing, stability studies, or mechanical & functional assessments, Cormica offers a range of advanced testing methodologies tailored specifically for the medical device industry. Our team of experts work closely with you to identify the most appropriate tests to validate your product’s integrity.

Pre-filled Syringes, Auto-Injectors, Wearables, Respiratory & More
Design Verification Testing
Design Validation Testing
- Tensile & Compression Tests
- Pressure & Vacuum Tests
Needle Injection Systems
Parental Containers
- ISO 11608-3
- ISO 8871-5
- ISO 11040-8
- USP 1207
- Dye Solution Tightness
- Trace Gas Detection
Physical, Functional and Mechanical Testing
- Tensile & Compression Tests
- Pressure & Vacuum Tests
- Flow Rate
- Attachment & Detachment Forces
- Pre-Conditioning
- ISO 80369
- Safety Feature Confirmation
- Sharps Bins
- ISO 23907-1
- Torque & Axial Forces
- Penetration Tests
Device Stability Studies
- Real Time & Elevated Conditions
- ISO 11608
- Tensile & Compression Tests
- Attachment & Detachment Forces
- Torque & Axial Forces
The timeline for physical and mechanical testing of medical devices depends on the specific tests required, such as tensile, compression, or flow rate tests. We offer flexible testing schedules, with standard testing turnaround times ranging from 1-2 weeks.
Mechanical testing and functional testing is crucial for a wide variety of medical devices, including needle injection systems, drug delivery devices, parental containers, and implantable technologies. Cormica specialises in testing to ensure device durability, performance, and compliance with industry standards like ISO 11608, ISO 11040, and USP 788.
For mechanical testing of medical devices, we follow several key ISO standards, including ISO 11608 for needle-based systems, ISO 11040 for prefilled syringes, and ISO 80369 for small-bore connectors. Our adherence to these international standards ensures your device meets global regulatory requirements.
Design verification testing ensures your medical device is built according to design specifications, while validation testing confirms it meets user needs and regulatory standards. At Cormica, we offer comprehensive physical and mechanical testing to support both design verification and validation processes, helping ensure your device is safe, effective, and compliant.