Quality Control Testing
Comprehensive Microbial Quality Control Testing Services for Pharmaceuticals and Medical Devices
Offering expert Microbial Analysis with test methods in Total Aerobic Counts, Yeast & Mould Counts, Specified Microorganisms, Potable Water, and Environmental Swabs
Accurate microbial quality control is crucial for ensuring the safety and compliance of pharmaceuticals, medical devices and combination products.
We adhere to standards set by authorities like the FDA, MHRA, and international pharmacopoeias.
As a GMP and GLP compliant lab, we offer precise testing methods that ensure product reliability and market credibility, minimising risks and safeguarding your brand’s reputation.
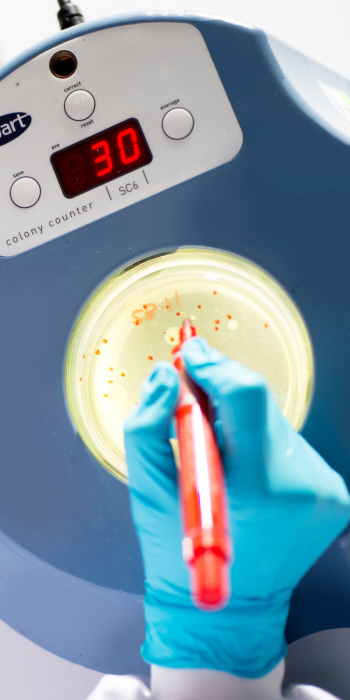
Your Partner in Microbial Quality Control Testing Services
Total Aerobic Microbial Counts
Determines the number of aerobic microorganisms in a specified quantity following either plate count method or membrane filtration method.
Total Yeast and Mould Counts
Quantifies the population of yeast and mould microorganisms in a specified quantity of sample by performing plate count method or membrane filtration method.

The method of testing is specifically chosen based on individual medical product requirements and specifications by adhering to the pharmacopeia regulations. Commonly medical devices and purified water samples are testing by membrane filtration.
Pharmaceutical products including oral, topical presentations and inhalation products usually follow TAMC / TYMC and absence of specified microorganism testing. Active pharmaceutical ingredient (API) containing samples are tested following plate count procedures.
Samples which we expect elevated bioburden in (for instance herbal samples) can be further diluted to obtain countable results.
Absence of Specified Microorganisms:
Determines the presence or absence of specified microorganisms
• Escherichia coli
• Salmonella spp
• Pseudomonas aeruginosa
• Staphylococcus aureus
• Bile tolerant gram-negative organisms
• Burkholderia cepacia complex
• Candida albicans
• Clostridia spp
The specific microorganism which is selected for absence testing within a product is sample-specific and the list is non-exhaustive. The factors which influence the decision of which objectionable microorganism to test for depends upon the:
- Nature of the product
- Starting material
- Manufacturing process
These tests are usually performed as a qualitative analysis, however with products that are known to have a high bioburden such as herbal products – they can be tested as a quantitative analysis.
The type of testing required will depend of the route of entry of the product. For example any topical presentations, such as creams and lotions, would usually be tested for the absence of Pseudomonas aeruginosa and Staphylococcus aureus.
Products that are going to be ingested or their route of entry is orally such as, tablets and capsules are tested for E.coli and Salmonella etc. as they will be digested inside the body. Introducing these types of bacterial could impose health implications for the consumer especially if the individual is immunocompromised or a high risk category.
Potable Water Testing
We can conduct testing on water from manufacturing facilities either as part of routine Environmental Monitoring sampling at your site, or through client samples sent directly to us.
Testing of potable water can determine the levels of microorganisms present, if growth is obtained, morphologies are then analysed by trained scientists to report whether growth is similar to that of the target organism.
The types of testing we can offer for potable water samples are as follows:
- TVC at 37C
- TVC at 22C
- Escherichia coli
- Total coliforms
- Pseudomonas aeruginosa
- Enterococci and other faecal organisms
- Clostridium perfringens

Enumeration of environmental swab testing
We can perform analysis of swab samples taken directly from a clients facility either from their routine Environmental Monitoring requirements or if they encounter a contamination issue and are swabbing their facility to investigate the source of contamination.
The swab is then transported in a suitable transport medium and received at Cormica to undergo analysis following plate count procedures.